Description
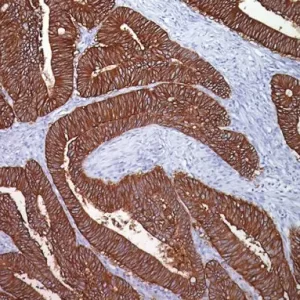
Prostate Specific Antigen antibody (PSA) is a chymotrypsin-like serine protease (kallikrein family) produced by the prostate epithelium. PSA can be used as a screening marker for differentiating high-grade prostate adenocarcinoma from high-grade urothelial carcinoma. PSA may also be a useful aid to confirm prostatic acinar cell origin in primary and metastatic carcinomas and to rule out non-prostatic carcinoma mimics. PSA can be a valuable tool in the diagnostic evaluation of metastatic adenocarcinoma of unknown primary origin in males.
SPECIFICATIONS
Specifications
| WEIGHT | N/A |
|---|---|
| DIMENSIONS | N/A |
| INTENDED USE | IVD |
| SPECIES REACTIVITY | Human |
| SOURCE | Rabbit Monoclonal |
| CLONE | EP109 |
| ISOTYPE | IgG |
| ANTIGEN | Prostate Specific Antigen |
| LOCALIZATION | Cytoplasmic |
| POSITIVE CONTROL | Prostate or Prostate Carcinoma |
DATASHEETS & SDS
| Download Data Sheet |
| Download RUO Data Sheet for International |
| Download SDS Sheet |
Regulatory Notice: Biocare’s IVD-labeled products comply with US-FDA regulation. Other regions may have additional requirements for such labeling, please contact your local distributor.
REFERENCES
1. Tazawa K, et. al. An immunohistochemical and clinicopathological study of gastrointestinal stromal tumors. Path Intl 49(6):500-505, 1999.
2. Siddiqui IA. et. al. Inhibition of CWR22Rnu1 tumor growth and PSA secretion in athymic nude mice by green and black teas. Carcinogenesis. 2006 Apr; 27(4):833-9. Epub 2005 Dec 29.
3. Ljung G, et. al. Characterization of residual tumor cells following radical radiation therapy for prostatic adenocarcinoma; immunohistochemical expression prostatespecific antigen, prostatic acid phosphatase, and cytokeratin 8. Prostate, May 1;31 (2):91-97, 1997.
4. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
5. National Committee for Clinical Laboratory Standards (NCCLS). Protection of laboratory workers from infectious diseases transmitted by blood and tissue; proposed guideline. Villanova, PA 1991;7(9). Order code M29-P.






Reviews
There are no reviews yet.