Description
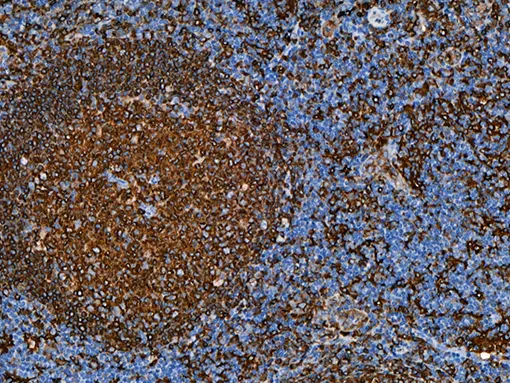
HLA-DR (Human Leukocyte Antigen – DR isotype) is a major histocompatibility complex (MHC) class II antigen presentation molecule, critical for the activation of lymphocytes and the coordinating of adaptive immune responses. HLA DR antigen is required for tumor-associated antigen recognition by CD4+ T cells. It is normally expressed on antigen-presenting cells including monocytes, macrophages, dendritic cells and B cells, but expression can be induced on epithelial cells and tumor cells in response to inflammatory conditions.15,16
SPECIFICATIONS
Specifications
| INTENDED USE | IVD |
|---|---|
| FORMAT | Concentrate, ONCORE Pro, Predilute, Q Series, UltraLine |
| VOLUME | 0.1 ml, 1.0 ml, 6.0 ml, 60 Tests, 7 ml |
| SOURCE | Mouse Monoclonal |
| SPECIES REACTIVITY | Human; others not tested |
| CLONE | TAL 1B5 |
| ISOTYPE | IgG1 |
| LOCALIZATION | Cell Membrane |
| POSITIVE CONTROL | Tonsil |
DATASHEET & SDS
REFERENCES
1. Kiernan JA. Histological and Histochemical Methods: Theory and Practice. New York: Pergamon Press 1981
2. Sheehan DC and Hrapchak BB. Theory and Practice of Histotechnology. St. Louis: C.V. Mosby Co. 1980
3. Clinical Laboratory Improvement Amendments of 1988: Final Rule, 57 FR 7163, February 28, 1992.
4. Shi S-R, Cote RJ, Taylor CR. J Histotechnol. 1999 Sep;22(3):177-92.
5. Taylor CR, et al. Biotech Histochem. 1996 Jan;71(5):263-70.
6. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
7. Clinical and Laboratory Standards Institute (CLSI). Protection of Laboratory Workers from Occupationally Acquired Infections; Approved Guideline-Fourth Edition CLSI document M29-A4 Wayne, PA 2014.
8. CLSI Quality Standards for Design and Implementation of Immunohistochemistry Assays; Approved Guideline-Second edition (I/LA28-A2) CLSI Wayne, PA USA (www.clsi.org). 2011
9. College of American Pathologists (CAP) Certification Program for Immunohistochemistry. Northfield IL. Http://www.cap.org (800) 323- 4040.
10. O’Leary TJ, Edmonds P, Floyd AD, Mesa-Tejada R, Robinowitz M, Takes PA, Taylor CR. Quality assurance for immunocytochemistry; Proposed guideline. MM4-P. National Committee for Clinical Laboratory Standards (NCCLS). Wayne, PA. 1997;1-46
11. Koretzik K, Lemain ET, Brandt I, and Moller P. Metachromasia of 3- amino-9-ethylcarbazole (AEC) and its prevention in Immunoperoxidase techniques. Histochemistry 1987; 86:471-478.
12. Nadji M, Morales AR. Immunoperoxidase, part I: the techniques and itspitfalls. Lab Med 1983; 14:767
13. Omata M, Liew CT, Ashcavai M, Peters RL. Nonimmunologic binding of horseradish peroxidase to hepatitis B surface antigen: a possible source of error in immunohistochemistry. AmJ Clin Path 1980; 73:626
14. Herman GE and Elfont EA. The taming of immunohistochemistry: the new era of quality control. Biotech & Histochem 1991; 66:194
15. Dunne MR, Phelan JJ, Michielsen AJ, et al. Characterising the prognostic potential of HLA-DR during colorectal cancer development. Cancer Immunol Immunother. 2020;69(8):1577-1588.
16. Matsushita K, Takenouchi T, Shimada H, et al. Strong HLA-DR antigen expression on cancer cells relates to better prognosis of colorectal cancer patients: Possible involvement of c-myc suppression by interferongamma in situ. Cancer Sci. 2006 Jan;97(1):57-63.






Reviews
There are no reviews yet.