Description
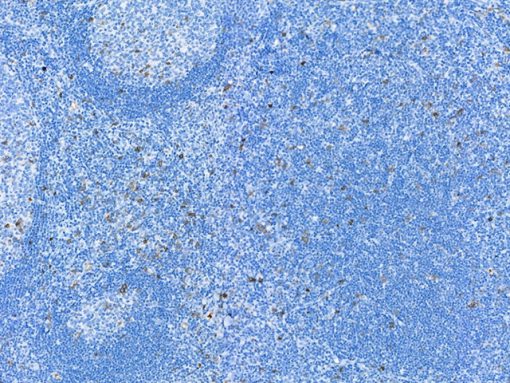
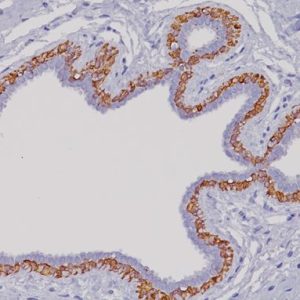
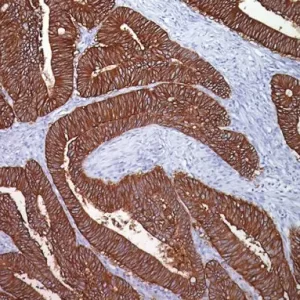
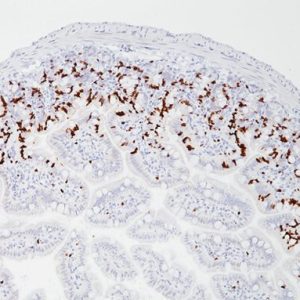
GITR (Glucocorticoid-induced TNF-R-related protein, TNFRSF18, CD357) is a co-stimulatory cell surface receptor constitutively expressed at high levels on T regulatory cells (Tregs), at intermediate levels on NK cells, and at low levels on naïve and memory T cells, and macrophage and dendritic cells (DCs) (1-2). Of all immune subsets studied, activated Tregs exhibit the highest level of GITR, an important distinction that becomes more apparent during the in vivo evaluation of GITR modulation (1-2). GITR ligand, GITRL (TNFSF18), is also a member of the TNF superfamily and is predominantly expressed by activated antigen presenting cells (APCs), including DCs, macrophage and activated B cells (2-4). The expression of both GITR and GITRL are not limited to hematopoietic cells. For example, GITR has been reported to be expressed at intermediate levels on epidermal keratinocytes and osteoclast precursors, whereas GITRL has been detected at high levels on endothelial cells, particularly following exposure to type I IFN (2). Therefore, tonsillar squamous epithelia would be the internal positive control for GITR and endothelial cells would be internal positive control for GITRL. The expression of GITR by tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment has been found to be higher than levels expressed by peripheral lymphocytes, indicating local T cell activation (5). Agonizing agents of this pathway have been considered as a way to increase the immune antitumor activity, although the clinical utility of such agents depends on the presence of T cells in the tumor and the subset of TILs which may vary among different malignancies (6). Thus, selection of patients who will derive the most benefit from this therapy is still unclear. Immune-related adverse events should also be considered. Preclinical data suggests that GITR therapy appears to be better tolerated than anti-CTLA4 agents (5-6). GITR modulation in the preclinical models has shown promising antitumor activity via significant increase in effector T cells and decrease in Tregs (5). Several human monoclonal antibodies that agonize GITR are currently undergoing phase I clinical studies in various solid malignancies. Preliminary results demonstrate an acceptable safety profile without dose limiting toxicities (7-9).
SPECIFICATIONS
Specifications
| INTENDED USE | IVD |
|---|---|
| FORMAT | Concentrate, Predilute |
| VOLUME | 0.1 ml, 0.5 ml, 6.0 ml |
| SPECIES REACTIVITY | Human; others not tested |
| SOURCE | Rabbit Monoclonal |
| CLONE | CAL8 |
| ISOTYPE | IgG1 |
| ANTIGEN | Synthetic peptide derived from a region of the GITR protein |
| LOCALIZATION | Membranous/cytoplasmic |
| POSITIVE CONTROL | Tonsil |
DATASHEETS & SDS
REFERENCES
1. Shimizu J, et al. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nature Immunol. 2002; 3:135-42.
2. Nocentini G, Riccardi C. GITR: a modulator of immune response and inflammation. Adv Exp Med Biol. 2009; 647:156-73.
3. Krausz LT, et al. GITR-GITRL system, a novel player in shock and inflammation. Scientific World Journal. 2007; 7:533-66.
4. Hanabuchi S, et al. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptorligand (GITRL). Blood. 2006; 107:3617-23.
5. Dempke WCM, et al. Second- and third-generation drugs for immunooncology treatment-The more the better? Eur J Cancer. 2017; 74:55– 72.
6. Knee DA, et al. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer. 2016; 67:1–10.
7. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
8. Clinical and Laboratory Standards Institute (CLSI). Protection of Laboratory Workers from Occupationally Acquired Infections; Approved Guideline-Fourth Edition CLSI document M29-A4 Wayne, PA 2014.



Reviews
There are no reviews yet.