Description
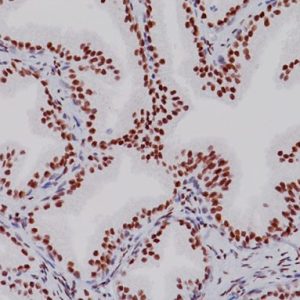
Cytomegalovirus Antibody (CMV) can precipitate and exacerbate gastrointestinal mucosal injury. Studies suggest that IHC performed on infected tissue with monoclonal antibodies directed against the CMV immediate early antigen is considered by most to be the current gold standard for diagnosis. This antibody is a mixture of two monoclonal antibodies that reacts with immediate early and early protein antigens in tissues infected with cytomegalovirus. Studies indicate that this antibody does not react with herpes virus or human papilloma virus (HPV). In the later stage of infection, a cytoplasmic reaction may be observed.
SPECIFICATIONS
Specifications
| WEIGHT | N/A |
|---|---|
| DIMENSIONS | N/A |
| INTENDED USE | ASR |
| SOURCE | Mouse Monoclonal |
| CLONE | DT10 + BC90 |
| ISOTYPE | IgG2a + IgG1 |
| ANTIGEN | Cytomegalovirus proteins |
| LOCALIZATION | Nuclear staining pattern with some cytoplasmic staining |
| POSITIVE CONTROL | CMV infected tissues |
DATASHEETS & SDS
| Download Data Sheet |
| Download ASR Data Sheet |
| Download RUO Data Sheet for International |
| Download SDS Sheet |
Regulatory Notice: Biocare’s IVD-labeled products comply with US-FDA and European IVDD regulation. Other regions may have additional requirements for such labeling, please contact your local distributor.
REFERENCES
1. Vago L, et al. Acta Neuropathol. 1996 Oct; 92(4):404-8.
2. Mills AM, et al. Am J Surg Pathol. 2013 Jul; 37(7):995-1000. 3. Kandiel A, Lashner B. Am J Gastroenterol. 2006 Dec; 101(12):2857-65.






Reviews
There are no reviews yet.