Description
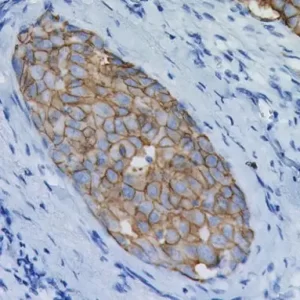
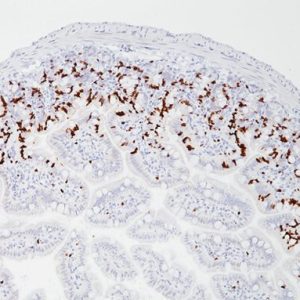
Uro-2 (CK20 + p53) is a primary antibody cocktail for the multiplex IHC identification of CK20 and p53 proteins in bladder. Studies have shown that in normal urothelium, the superficial umbrella cell layer shows reactivity for CK20 only; whereas, p53 nuclear staining is absent to focal. For urothelium with reactive atypia, particularly in cases with marked atypia, CK20 and p53 staining remain identical to those seen in normal urothelium. In cases of CIS, diffuse, strong cytoplasmic reactivity for CK20 and diffuse nuclear reactivity for p53 is observed throughout the urothelium. Most high-grade dysplasia stain with p53 when compared to low-grade dysplasia.
SPECIFICATIONS
Specifications
| WEIGHT | N/A |
|---|---|
| DIMENSIONS | N/A |
| INTENDED USE | IVD |
| SPECIES REACTIVITY | Human |
| SOURCE | Mouse Monoclonal, Rabbit Monoclonal |
| CLONE | Ks20.8 + EP9 |
| ISOTYPE | IgG2a + IgG |
| ANTIGEN | CK20 and p53 protein |
| LOCALIZATION | CK20 (Cytoplasmic): Brown p53 (Nuclear): Red [Optional] CD44 (Cell membrane, cytoplasmic): Purple (see optional protocol) |
| POSITIVE CONTROL | p53 positive bladder or colon cancers |
DATASHEETS & SDS
| Download DS Data Sheet |
| Download RUO Data Sheet for International |
| Download SDS Sheet |
Regulatory Notice: Biocare’s IVD-labeled products comply with US-FDA and European IVDD regulation. Other regions may have additional requirements for such labeling, please contact your local distributor.
REFERENCES
1. Russo S, et al. A useful panel in proliferation urothelial lesions: an analysis of cytokeratin 20, p53, CD44 and Ki-67 antigens. Pathologica. 2007 Apr; 99(2):46-9.
2. McKenney JK, et al. Discriminatory immunohistochemical staining of urothelial carcinoma in situ and non-neoplastic urothelium: an analysis of cytokeratin 20, p53 and CD44 antigens. Am J Surg Pathol. 2001 Aug; 25(8):1074-8.
3. Sun W, Zhang PL, Herrera GA. p53 protein and Ki-67 overexpression in urothelial dysplasia of bladder. Appl Immunohistochem Mol Morphol. 2002 Dec; 10(4):327-31.
4. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
5. Clinical and Laboratory Standards Institute (CLSI). Protection of Laboratory workers from occupationally Acquired Infections; Approved guideline-Third Edition CLSI document M29-A3 Wayne, PA 2005





Reviews
There are no reviews yet.