Description
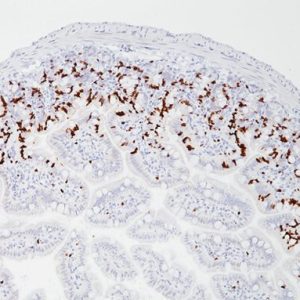
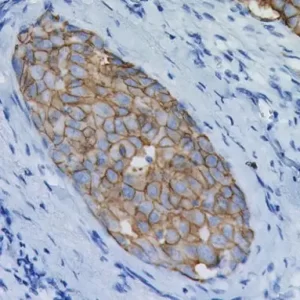
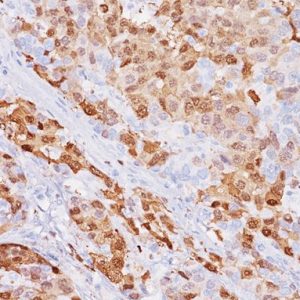
Mesothelin is a cell surface glycoprotein that elicits T cell responses, first described as the antigenic target of the K1 monoclonal antibody, generated by using an ovarian cell line as immunogen (1,7). In normal tissues, mesothelin expression is largely restricted to mesothelial cells. However, immunoreactivity for this marker has also been reported in some epithelial cells of the trachea, tonsil, kidney, and fallopian tube (1). Mesothelin is one of the most sensitive markers for mesothelioma, and it has been reported to be expressed in all or nearly all epithelioid mesotheliomas as well as in some carcinomas, particularly those arising in the ovary (serous carcinomas) and pancreas (1-7). Epithelioid mesotheliomas and adenomatoid tumors invariably express mesothelin, and the reaction is usually strong and diffuse and occurs along the cell membrane. This is in contrast to sarcomatoid mesotheliomas, which are usually negative for this marker (6,8). Among carcinomas, most pancreatic adenocarcinomas (86%-100%) and nonmucinous carcinomas of the ovary, including serous carcinomas (93%-100%), clear cell carcinomas (43%-75%), and transitional cell carcinomas (100%), have been reported to be mesothelin positive (8). Approximately 40% to 50% of pulmonary adenocarcinomas and 15% to 30% of squamous cell carcinomas of the lung have also been reported to express this marker in a focal and cytoplasmic staining pattern, in contrast to mesotheliomas’ membranous pattern (4,5,8). Even with the low specificity for mesothelioma, the common strong membranous reactivity in epithelioid mesotheliomas should be regarded as a strong indication against the diagnosis of mesothelioma when a negative stain is achieved (3). In addition, because mesothelin expression has been reported to be either negative or rarely weakly positive in some carcinomas, such as renal cell carcinomas, it may be included in the panel of markers used to distinguish these tumors from epithelioid mesotheliomas (5,8).
SPECIFICATIONS
Specifications
| BY LETTER | M |
|---|---|
| ANTIGEN | Mesothelin |
| CLONE | MSLN-15C11 |
| FORMAT | Concentrate, Predilute |
| INTENDED USE | IVD |
| ISOTYPE | IgG1/kappa |
| LOCALIZATION | Membranous for mesotheliomas; cytoplasmic for carcinomas |
| POSITIVE CONTROL | Lung and fallopian tube |
| SOURCE | Mouse Monoclonal |
| SPECIES REACTIVITY | Human; others not tested |
| VOLUME | 0.1 ml, 0.5 ml, 6.0 ml |
DATASHEETS & SDS
REFERENCES
1. Chang K, et al. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992 Feb 1;50(3):373-81.
2. Ordóñez NG. The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol. 2003 Aug;27(8):1031-51.
3. Ordóñez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003 Mar;16(3):192-7.
4. Frierson HF Jr, et al. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum Pathol. 2003 Jun;34(6):605 -9.
5. Ordóñez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003 Nov;27(11):1418-28.
6. Ordóñez NG. The diagnostic utility of immunohistochemistry in distinguishing between mesothelioma and renal cell carcinoma: a comparative study. Hum Pathol. 2004 June;35(6):697-710.
7. Tchou J, et al. Mesothelin, a novel immunotherapy target for triple negative breast cancer. Breast Cancer Res Treat. 2012 Jun;133(2):799-804.
8. Ordóñez NG. Application of immunohistochemistry in the diagnosis of epithelioid mesothelioma: a review and update. Hum Pathol. 2013 Jan;44(1):1-19.
9. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
10. Clinical and Laboratory Standards Institute (CLSI). Protection of Laboratory Workers from Occupationally Acquired Infections; Approved Guideline-Fourth Edition CLSI document M29-A4 Wayne, PA 2014




Reviews
There are no reviews yet.